Fagron Sterile Services US Launches RFID enabled products to support patient safety and hospital inventory management at the first annual Medication Intelligence Summit and are joined by their project partners Kit Check tag the virtual summit which brings together healthcare executives, experts and pharmacists to discuss hospital operational trends.
Announcing the commercial availability of select presentations of Radio Frequency Identification (RFID) enabled ready-to-administer operating room syringes, Fagron Sterile Services collaborated with Kit Check to create the RFID tagged syringes.
Following our recent FSS facility expansion announcement, partnering with Kit Check to support patient safety and medication management is yet another exciting example of our commitment to producing reliable, high-quality medication solutions for our customers and the patients they serve.
Andrew Pulido, President, Fagron North America.
Medications labeled with RFID tags at the manufacturer level ensures that there is item-level visibility of drug products throughout their dynamic lifecycle. This means better patient safety, optimized inventory for hospitals, cost savings, and dramatically increased workflow simplicity and efficiency.
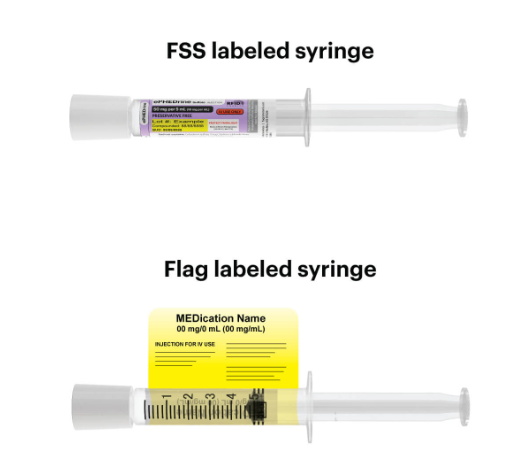
RFID tags, in combination with the Kit Check technology, store information for each dose, including expiration date, lot number, NDC, and refrigeration date, so restocking, inventory management, and managing shortages and recalls is as seamless as possible. COVID-19 has continued to shine a light on the importance of item-level inventory management in the hospital, with Kit Check customers well poised to deal with the difficulty inventory challenges the pandemic has caused.
Visit Fagron Sterile Kit Check for more information.
Send us your News
If you have a story you think we would be interested in relating to RFID. A product launch, a new RFID technology or a successful RFID implementation project, please do not hesitate to send us your press release.